Iron Infusion in Haemodialysis Study: Intravenous Iron Polymaltose For Indigenous Patients with High Ferritin Levels on Haemodialysis; A Prospective, Open-Label, Blinded Endpoint, Randomised Controlled Trial
Summary:
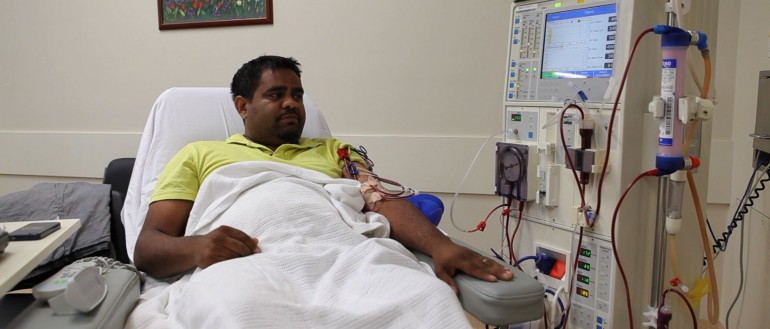
INFERR will assess the safety and effectiveness of giving intravenous iron to Aboriginal and Torres Strait Islander patients on haemodialysis with anaemia, high ferritin (a marker of both iron levels and inflammation) and low blood levels of iron.
This treatment will potentially benefit the majority of Aboriginal and Torres Strait Island haemodialysis patients in the Northern Territory (NT) who are currently receiving routine iron treatment, but for whom the safety and efficacy of treatment remains poorly defined.
A recent, large scale, multicenter, prospective, open label, blinded endpoint clinical trial from the UK (PIVOTAL) confirmed the safety and efficacy of high dose IV Iron (400mg of iron once a month). This provides the best evidence for a standard treatment as the results showed that this regimen was not inferior to the reactive low-dose IV iron therapy. Most importantly, there were no differences in adverse effects such as increased risk of infections. However, the exclusion of patients with high ferritin makes the results of this trial difficult to extrapolate to our (NT) dialysis patients given that the majority (> 80%) would have a ferritin higher than 700µg/L. This re-enforces the need for a clinical trial to assess the safety and efficacy of IV iron in haemodialysis patients within the NT with high ferritin.
This study will generate evidence to underpin a part of routine care and to ensure we use IV iron appropriately for the benefit of Aboriginal and Torres Strait Island dialysis patients in the NT.
How to get involved in this study:
If you are 18 years of age or older, have been receiving haemodialysis for at least three months and are Aboriginal or Torres Strait Islander you may be eligible for this study.
Chief investigators:
Project manager:
- Contact Jane Nelson
Project dates:
The project commenced in January 2019 and is due for completion in 2023.
Funder:
- National Health and Medical Research Council