CAMERA2
Combination Antibiotic Therapy for Methicillin Resistant Staphylococcus Aureus infection.
PhD - Opportunity
- We are looking for EOI for an ID / microbiology fellow to conduct follow-on studies from CAMERA2 as part of a PhD, click here for more details.
CAMERA2 - Research Focus
The CAMERA2 study group would like to make available access to the CAMERA2 clinical trial de-identified data and /or bacterial isolate collection to other interested researchers.
All applications will be considered by the CAMERA2 Trial Management Committee and approval will be based on the criteria stipulated in the submitted application. Please note that the trial investigators already have a number of planned sub-studies and will determine which new sub-studies will be prioritised. Applications for sub-studies can be commenced now, but resulting manuscripts will only be able to be submitted following acceptance of the primary trial outcome manuscript.
Please note you must liaise with a CAMERA2 study group member listed who can assistance you in completion of the application. If you are unfamiliar with any of the members then please refer all correspondence to Prof Joshua Davis.
CAMERA2 - Trial Design:
CAMERA2 was an investigator-initiated, multi-centre, parallel group, open-label, randomised controlled trial powered for superiority, which compared combination antibiotic therapy with standard antibiotic therapy in adults with MRSA bacteraemia.
Consented participants were randomised on day 1 to receive standard therapy alone, or standard therapy plus 7 days of IV β-lactam. Data was captured from randomisation (day 1) until 90 days post randomisation from the participant’s medical records and medication charts (electronic and paper). Whilst the participant remained an inpatient, their medical records were reviewed at least weekly until discharge or the 90 day time point whichever occurred first. Information collected includes blood test results (FBC, EUC, LFTs, CRP, blood cultures and vancomycin levels), relevant comorbidities, baseline SOFA score, baseline PITT bacteraemia, vital status, antibiotic administration, nephrotoxins, echocardiography, MRSA positive cultures and any other hospital admissions if discharged within the 90 days. The attached CRFs (1 to 4) provide data collection specifics.
Blood culture positive bacterial isolates from the index (first positive blood culture taken from this patient for this current hospital admission) through to Day 90 were salvaged from participating sites, please note not all isolates were able to be retrieved. These are also available for use.
Note:
Please see the source standard data collection tools :
- CAMERA2 Study Protocol
- CRF 1: Eligibility criteria data collection
- CRF 2: Baseline data collection
- CRF 3: Daily data collection (day 1 until day 7)
- CRF 4: Follow-up data collection (day 8 until day 90)
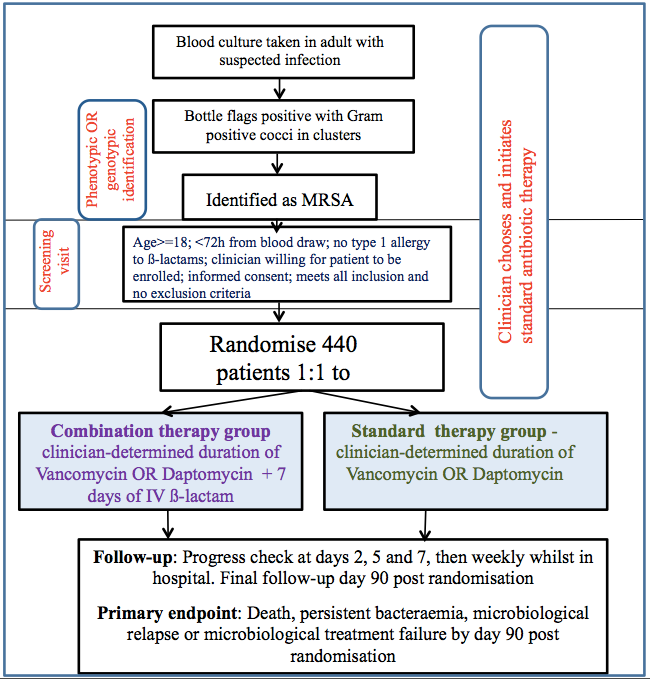
358 Participants were enrolled into the study over a period of 4 years.
Contacts:
- Prof Joshua Davis - Coordinating Chief Investigator - joshua.davis@menzies.edu.au
- A/Prof Steven Tong - Coordinating Chief Investigator - Steven.Tong@mh.org.au
- Jane Nelson - Project Manager - jane.nelson@menzies.edu.au - Phone: +61 0 8 8946 8522

